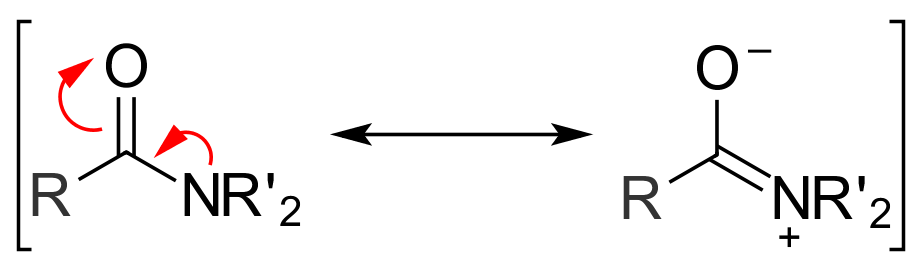
An amide is a useful team in which a carbonyl team is linked with the nitrogen atom by a single bond.
Amide is also referred to as a by-product of carboxylic acids and has an OH category of COOH put back by an NH2, NR2, and NHR of an amine.
To check the amide hydrolysis process, please visit the link.
What’s Hydrolysis?
Hydrolysis is a chain reaction of the communication of chemicals with water, leading to the decay of both water, well as the material.
Hydrolysis of Amide
Amide stands up to hydrolysis; even after extended home heating, amide does not engage with water molecules to break down. Nevertheless, hydrolysis of amide is not impossible. It engages with water particles either in a fundamental or an acidic medium.
In an acidic tool, amide interacts with the water particle to offer a carboxylic acid, as well as the salt of amine salt or ammonia.
In a basic medium, amide communicates with the water particle to provide a carboxylic acid, as well as the amine salt or salt of ammonia.
In the human body, amide hydrolysis is catalyzed by enzymes.
Sorts of Amide Hydrolysis
Amide connects with a water molecule in two possible means, i.e.,
- Base Catalyzed Amide Hydrolysis
- Acid-Catalyzed Amide Hydrolysis
Acid-Catalyzed Amide Hydrolysis
In an acidic medium, amide communicates with the water molecule to offer a carboxylic acid and the amine salt or salt of ammonia.
System:
- Protonation of amide carbonyl
We will activate the amide because we only have a weak nucleophile, as well as a poor electrophile. Protonation of the amide carbonyl makes it extra electrophilic.
- Nucleophilic addition
Lone pairs of oxygen would assault the carbonyl group; the electron would relocate towards the oxonium ion, creating a tetrahedral intermediate.
- Proton transfer
Oxygen loses a proton to neutralize the rate.
- Proton transfer
Proton attacks -NHR’s team to make sure that it can leave easily.
- Removal of R’- NH2
Lone pairs of oxygen strike the carbon atom to push out the R’-NH2 group.
- Deprotonation of oxonium ion
Oxygen loses a proton to neutralize the rate.
Base Catalyzed Amide Hydrolysis
In a standard medium, amide interacts with the water molecule to give a carboxylic acid, as well as the amine salt or salt of ammonia.
System:
- In base-catalyzed amide hydrolysis, the amide is warmed with boiling liquid KOH or NaOH.
- The nucleophilic hydroxide ion contributes to the carbonyl carbon to create a tetrahedral intermediate.
- Proton attacks the NR2 team.
- Oxonium ion assaults the carbon atom.
- HNR2 is cleaved.
To read about what is a peptide bond, please click on the link.